Periodic Table with Charges: Comprehensive Guide for Students
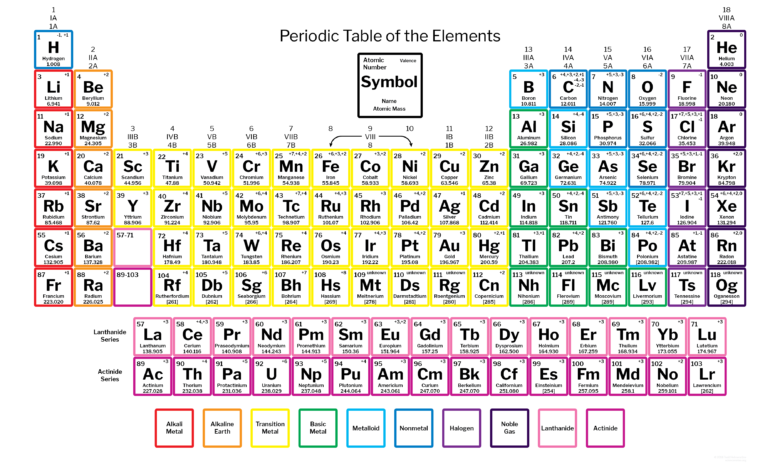
Introduction
The periodic periodic table with charges table is the backbone of chemistry, a powerful tool that organizes all known elements in a logical structure. For students, scientists, and enthusiasts alike, understanding the periodic table unlocks the mysteries of chemical reactions and bonding. A critical aspect of this understanding lies in grasping the concept of charges. Charges, whether positive or negative, define how elements interact to form compounds, govern their reactivity, and explain their role in countless chemical processes.
In this comprehensive guide, we’ll delve into the world of the periodic table with charges. From understanding what charges are to exploring group-specific trends, applications, and practical learning tools, this guide covers it all. Let’s simplify chemistry one charge at a time!
1. Understanding Charges in the Periodic Table

1.1 What Are Charges?
Charges are the result of an atom gaining or losing electrons, creating an imbalance between protons (positive) and electrons (negative). If an atom loses electrons, it becomes positively charged (cation). Conversely, gaining electrons makes it periodic table with charges negatively charged (anion). These charges are not arbitrary; they reflect an element’s natural tendency to stabilize its electron configuration, often by mimicking the nearest noble gas.
For example, sodium (Na) loses one electron to form a +1 charge, achieving the electron configuration of neon. Chlorine (Cl), on the other hand, gains periodic table with charges one electron to achieve the configuration of argon, resulting in a -1 charge. This complementary nature of charges drives the formation of ionic compounds.
1.2 How Charges Are Determined
The charge of an element is determined by its electron periodic table with charges configuration, specifically the number of valence electrons. Elements in Groups 1 and 2 typically lose electrons to achieve a stable configuration, resulting in positive charges. Nonmetals, especially those in Groups 15-17, often gain electrons to complete their outer shell.
Transition metals are unique because they can exhibit periodic table with charges multiple charges due to their ability to lose different numbers of electrons from their d-orbitals. For instance, iron (Fe) can form Fe²⁺ or Fe³⁺ ions depending on the reaction context.
1.3 Periodic Trends Affecting Charges
Periodic trends, such as electronegativity and ionization periodic table with charges energy, play a significant role in determining charges. As you move across a period, elements become more electronegative, meaning they are more likely to gain electrons and form negative charges. Conversely, elements on the left side of the periodic table, with low ionization energy, tend to lose electrons and form positive charges.
Understanding these trends is key to predicting how elements periodic table with charges will behave in chemical reactions, making charges an essential concept in mastering chemistry.
2. Groups and Their Common Charges
2.1 Alkali Metals (Group 1)
The alkali metals, including lithium, sodium, and potassium, are known for their +1 charge. This is because they each have one valence electron, which they readily lose to achieve a stable configuration. Their reactivity increases as you move down the group, making cesium and francium some of the most reactive elements.
These metals are essential in many industrial applications, such as sodium in table salt (NaCl) and potassium in fertilizers. Their consistent charge makes them predictable in reactions, simplifying chemical equations.
2.2 Alkaline Earth Metals (Group 2)
Alkaline earth metals like magnesium and calcium typically form +2 charges. With two valence electrons, they achieve stability by losing both. These elements are crucial for biological systems, such as calcium in bones and magnesium in enzymes.
Magnesium’s +2 charge also makes it ideal for lightweight alloys, while calcium’s reactivity underpins its use in cement and plaster.
2.3 Transition Metals
Transition metals are fascinating because they don’t conform to a single charge. For example, copper can form Cu⁺ or Cu²⁺ ions, while manganese exhibits charges ranging from +2 to +7. This variability stems from their d-orbitals, which allow electrons to be lost or shared in multiple ways.
Their versatility makes transition metals indispensable in industrial catalysts, electronic devices, and biological systems.
2.4 Halogens (Group 17)
Halogens like fluorine, chlorine, and bromine typically carry a -1 charge because they gain one electron to complete their octet. Their high electronegativity makes them highly reactive, particularly with alkali and alkaline earth metals, forming ionic compounds like NaCl.
In addition to their role in salt formation, halogens are used in water purification, disinfectants, and the production of organic compounds.
2.5 Noble Gases (Group 18)
Noble gases are the exception, as they rarely form charged ions. Their full valence shells make them chemically inert. However, under extreme conditions, some noble gases like xenon can form compounds and exhibit positive charges.
3. Practical Applications of the Periodic Table with Charges
3.1 Predicting Compound Formation
Understanding charges helps chemists predict how elements combine to form compounds. Sodium (+1) and chlorine (-1), for instance, form the ionic compound NaCl, with the charges perfectly balancing each other.
This predictive power extends to more complex molecules, such as magnesium sulfate (Mg²⁺ and SO₄²⁻), making charges invaluable in studying chemical bonding.
3.2 Balancing Chemical Reactions
Charges are crucial in balancing chemical equations. For example, in the reaction of aluminum with oxygen, Al³⁺ ions combine with O²⁻ ions to form Al₂O₃. Without understanding charges, balancing such equations would be daunting.
3.3 Applications in Real-World Chemistry
From powering batteries with lithium ions to treating medical conditions with calcium supplements, the concept of charges finds countless applications. In industries, electroplating relies on the controlled movement of charged ions, while biological systems depend on charged particles like sodium and potassium for nerve signaling.
4. Learning Tools for Mastering the Periodic Table with Charges
4.1 Visual Aids and Diagrams
Color-coded periodic tables highlighting common charges are excellent study tools. They provide an instant visual reference, helping learners quickly identify patterns and trends.
4.2 Online Tools and Apps
Interactive apps and websites allow users to practice predicting charges, visualize ion formation, and even simulate chemical reactions. Tools like ChemDoodle and Ptable are highly recommended for interactive learning.
4.3 Study Tips and Tricks
Mnemonics like “LiNa K Rubs Cats Fur” (Li, Na, K, Rb, Cs, Fr) for Group 1 metals help memorize common charges. Regular practice with ionic equations reinforces this understanding, making it second nature.
Conclusion
The periodic table with charges is more than just a chart; it’s a roadmap to understanding chemical behavior. By mastering the concept of charges, you unlock the ability to predict reactions, balance equations, and appreciate the role of elements in everyday life. With the right tools and study strategies, navigating the periodic table becomes an exciting journey into the heart of chemistry.

